Hydrochloric Acid Reacts With Barium Hydroxide According to the Equation
The equation for this reaction is Ba OH2 2 HCl BaCl2 2 H2O. What is the word equation for magnesium hydroxide plus hydrochloric acid.
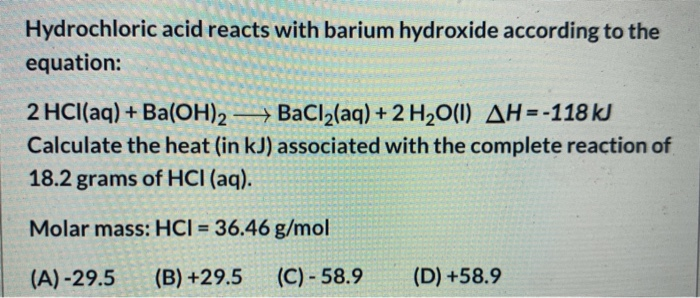
Solved Hydrochloric Acid Reacts With Barium Hydroxide Chegg Com
Barium sulfate BaSO 4 is made by reacting barium hydroxide and other barium sources with sulfuric acid and has a long history as a translucent white pigment.

. HClaqBaOH2aq ----- BaCl2aqH2Ol and also given Delta H -118kJ. Hydrochloric acid reacts with barium hydroxide according to the equation. 2 HCl aq BaOH2 aq.
Check the balance Barium hydroxide react with nitric acid to produce barium nitrate and water. Barium hydroxide hydrochloric acid balanced equation. An acid-base reaction is called a neutralization reaction It consists of the transfer of a hydroxide ion H from the acid to the base.
The balanced equation is. This means that the reaction consumes twice as many moles of hydrochloric acid than moles of barium hydroxide. Magnesium hydroxide and hydrochloric acid react to produce water and magnesium chloride.
Hydrochloric acid reacts with barium hydroxide according to the equation. In a balanced equation the number of atoms of each element will remain equal. 140 g BaOH 2 1 mol BaOH 2 1 mol BaCl 2 2083 g BaCl 2 1713 g BaOH 2 1 mol BaOH 2 1 mol BaCl 2 __17 g BaCl 2 ____ b.
2 HCl aq Ba OH2 aq BaCl2 aq 2 H2O l ΔH -118 kJ Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq. 2 hcl aq baoh2 aq bacl2 aq 2 h2o l δh -118 kj calculate the heat in kj associated with the complete reaction of 109 grams of hcl aq. BaCl2 aq 2 H2O l H -118 kJ Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq.
2 HClaq BaOH2 - BaCl2aq 2 H2O1 AH-118 kJ Calculate the heat in kJ associated with the complete reaction of 182 grams of HClaq. Chemistry questions and answers. Hydrochloric acid reacts with barium hydroxide according to the equation.
B a 2 H C l B a C l 2 H 2 When barium reacts with hydrochloric acid it will form barium chloride with the evolution of hydrogen gas. Nonomura in Cosmetic Science and Technology 2017 142115 Barium Sulfate. Use the molarities and volumes of the two solutions to determine how many moles of each youre mixing.
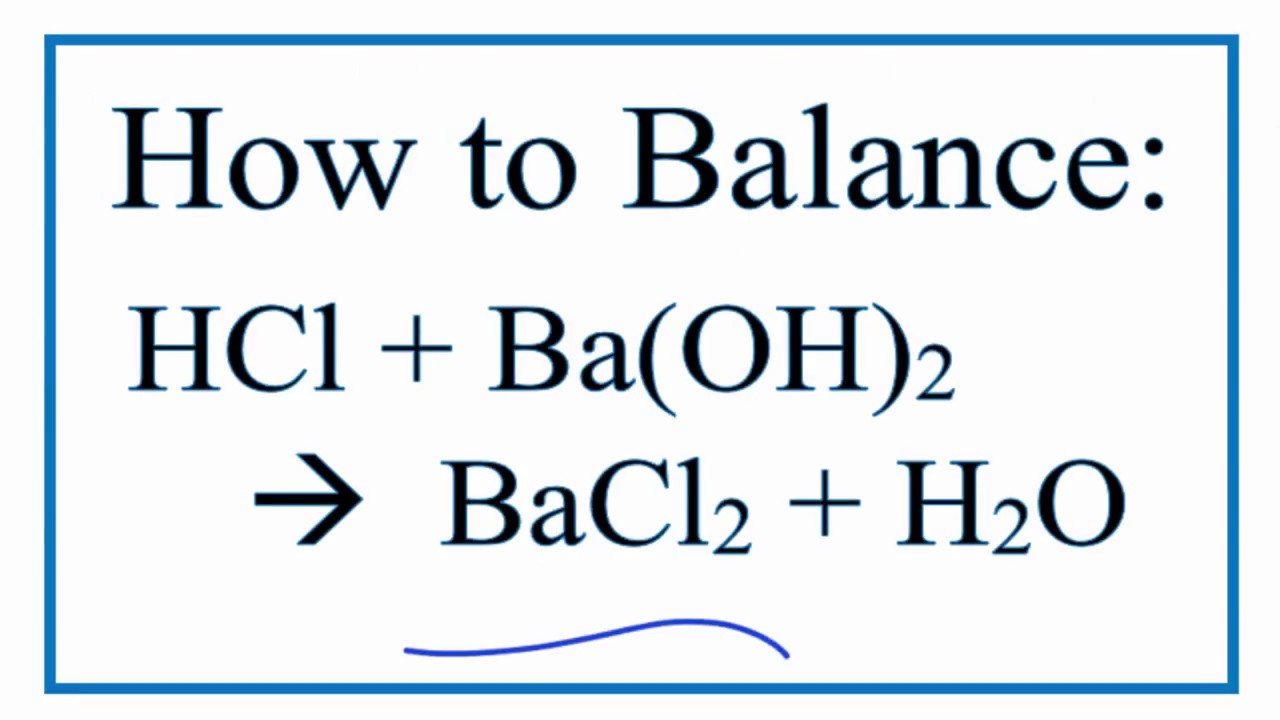
To balance HCl BaOH2 BaCl2 H2O. Write a balanced equation for the reaction below. Hydrochloric acid reacts with barium hydroxide according to the equation.
In this video well balance the HCl BaOH2 BaCl2 H2O and provide the correct coefficients for each compound. 2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The reaction consumes moles of hydrochloric acid. 2 HCl aq BaOH2 aq BaCl2 aq 2 H2O l Δ H -118 kK Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq.
The balanced equation for this reaction is. Mgs 2 HClaq -- MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gasThe flammability of hydrogen gas can be demonstrated by carefully holding a match or fireplace. 150 g BaOH 2 1 mol.
Barium sulfate can be formed in various shapes such as planar starred or spherical structures depending on the formulation condition. Ba OH2 is a strong base while HCl is a strong acid. Magnesium reacts with hydrochloric acid according to the equation.
Combustion reaction- A substance reacts with oxygen emitting heat forming an. None of these above. Calcium hydroxide CaOH2 acetic acid HC2H3O2 nitric acid HNO3 perchloric acid HClO4.
See the answer See the answer done loading. Hydrochloric acid reacts with barium hydroxide according to the equation. 2HCL BaOH2 BaCL2 2H2O.
How many grams of hydrochloric acid are needed to react with 150g of barium hydroxide. According to the following reaction how many moles of barium chloride will be formed upon the complete reaction of 0307 moles hydrochloric acid with excess barium hydroxide. Here 1 mole of barium reacts with 2 moles of hydrochloric acid to produce 1 mole of barium chloride and 1 mole of hydrogen gas.
Hydrochloric acid aq barium hydroxide aq barium chloride aq water l 1 See answer. What mass of barium chloride will form if 140g of barium hydroxide reacts with excess hydrochloric acid. 2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The reaction consumes moles of hydrochloric acid.
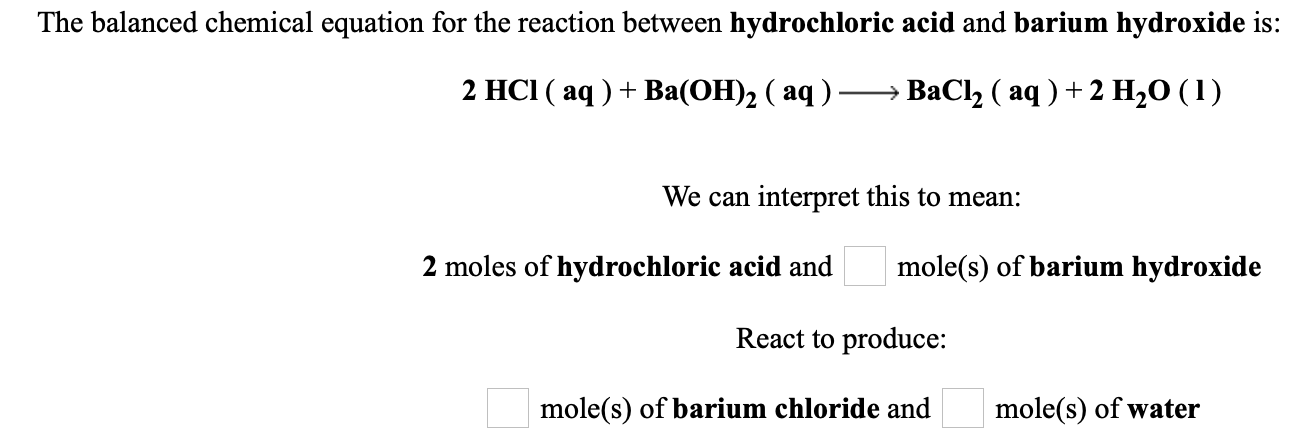
Barium hydroxide hydrochloric acid balanced equation. Notice that you have a 1colorred2 mole ratio between barium hydroxide and hydrochloric acid. Hydrochloric acid reacts with barium hydroxide according to the equation.
The balanced equation for this reaction is. When barium hydroxide is titrated with hydrochloric acid two molecules of hydrochloric acid combine with one molecule of barium hydroxide to produce one molecule of barium chloride and two molecules of water.

How To Write The Net Ionic Equation For Hcl Ba Oh 2 Bacl2 H2o Youtube
How To Balance Hcl Ba Oh 2 Bacl2 H2o Hydrochloric Acid Plus Barium Hydroxide Youtube

Ba Oh 2 Hcl Bacl2 H2o Balanced Chemical Equation
Solved The Balanced Chemical Equation For The Reaction Chegg Com
No comments for "Hydrochloric Acid Reacts With Barium Hydroxide According to the Equation"
Post a Comment